Abstract
Background
The prognosis for older adults diagnosed with acute myeloid leukemia (AML) is poor. The response to conventional chemotherapy in these patients is frequently brief, and long-term survival is largely limited to those who undergo allogeneic hematopoietic stem cell transplantation (HSCT). However, mortality after transplantation HSCT is also high, with deaths attributable both to relapsed AML and to transplant-related complications. There is thus a critical need for strategies that can reliably discern older AML patients who are most likely to benefit from current transplantation approaches and those for whom novel approaches should be developed.
We have previously shown that genetic characteristics can divide AML patients into three clinically distinct categories: (1) TP53 -mutated AML; (2) "secondary-type" AML, defined by mutations in any of eight genes with high specificity for the presence of antecedent myelodysplastic syndrome; and (3) "de novo" type AML, defined by absence of TP53 and secondary-type mutations. We showed that older patients with de novo-type AML have improved response to standard induction chemotherapy and significantly superior event-free survival than those with either secondary-type or TP53- mutated AML. Since disease burden prior to HSCT has been linked to post-HSCT outcomes, we hypothesized that diagnostic ontogeny classification would be associated with relapse and survival among older AML patients receiving HSCT in first complete remission.
Methods
We identified AML patients age 60 or older who underwent HSCT at Dana-Farber Cancer Institute (DFCI; Boston, MA) in first complete remission between 2012-2016, for whom next-generation sequencing of the diagnostic AML sample was available. Based on targeted gene panel sequencing of recurrent myeloid driver alterations, we classified AML as TP53 -mutated, secondary-type (SRSF2, SF3B1, U2AF1, ZRSR2, BCOR, ASXL1, EZH2, or STAG2 mutations), or de novo type (neither TP53 mutation nor secondary-type mutations). We extracted data on clinical outcomes from the medical record and assessed the impact of diagnostic AML genetics on overall survival and cumulative incidence of relapse following HSCT.
Results
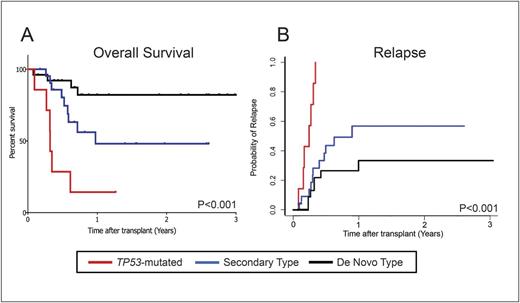
Fifty-five patients met inclusion criteria, with a median age of 67 (range 60-76). All had received intensive induction chemotherapy; nine (16.4%) received myeloablative conditioning regimens, while the remainder received reduced intensity regimens. Of the 55 patients, 13% (n=7) had TP53 mutations, 40% (n=22) had secondary-type mutations, and 47% (n=26) had de-novo type mutations. Patients with de-novo type mutations had significantly superior 2-year overall survival than those with secondary-type mutations, who in turn had significantly superior survival than those with TP53 mutations (82.2% versus 48.1% versus 14%, p<0.001 for all comparisons, Figure 1A). These differences reflected a significant difference in the 2-year cumulative incidence of relapse following HSCT (100% for TP53 versus 56.7% for secondary-type versus 33.4% for de-novo type mutations, p<0.001 for all comparisons, Figure 1B).
Conclusion
In our cohort of older AML patients who underwent HSCT in first complete remission after intensive induction chemotherapy, genetic ontogeny classification at the time of diagnosis was significantly associated with overall survival and rate of relapse after HSCT. Our findings, which merit further evaluation in a larger cohort, suggest that genetic classification of ontogeny can form the basis of a robust prognostic model that accurately predicts post-HSCT outcomes in older AML patients.
Koreth: Amgen Inc.: Consultancy; Millennium Pharmaceuticals: Research Funding; Prometheus Labs: Research Funding; Kadmon Corp: Membership on an entity's Board of Directors or advisory committees; Takeda Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Nikiforow: Kite Therapeutics: Membership on an entity's Board of Directors or advisory committees. Armand: Sigma Tau: Research Funding; Sequenta/Adaptive: Research Funding; Affimed: Research Funding; Infinity: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Genmab: Consultancy; Roche: Research Funding; Pfizer: Consultancy, Research Funding; Merck & Co., Inc.: Consultancy, Research Funding; Tensha: Research Funding; Otsuka: Research Funding. Antin: Gentium SpA/Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Lindsley: MedImmune: Research Funding; Jazz Pharmaceuticals: Consultancy; Takeda Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract